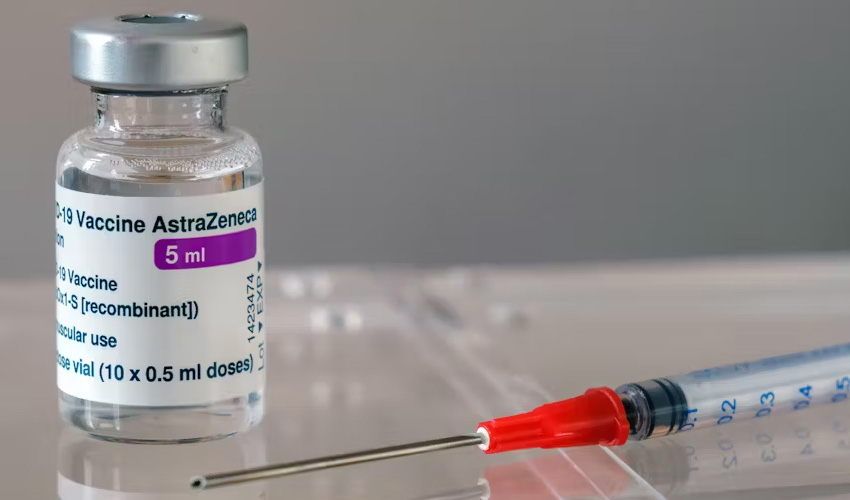
Following the Covid-19 pandemic, AstraZeneca announced on Tuesday that it had begun the global withdrawal of its vaccine because there was a "surplus of available updated vaccines".
Additionally, the company announced that it would remove the marketing authorizations for the vaccine Vaxzevria in Europe.
The company stated that "as multiple, variant COVID-19 vaccines have since been developed, there is a surplus of available updated vaccines", so there is less demand for Vaxzevria, which is no longer produced or supplied.
Also Read: AstraZeneca admits side effects of Covid vaccine
The Anglo-Swedish pharmaceutical company reportedly acknowledged in court filings that the vaccine has adverse effects, including low blood platelet counts and blood clots.
The Telegraph, which broke the story first, stated that the company applied on March 5 to withdraw the vaccine, and it took effect on May 7.
Following a slowdown in growth as sales of Covid-19 medications decreased, London-listed AstraZeneca started to expand into respiratory syncytial virus vaccines and obesity medications through a number of acquisitions last year.